Abstract
Background: Heterozygosity for a beta-thalassemia mutation is clinically insignificant; however, microcytosis and mild anemia are oftenpresent. Rare mutations in the proximal half of the coding sequence for the beta globin gene (HBB) have been shown to cause an apparent autosomal dominant beta thalassemia that is associated with more severe anemia. These mutations often produce a frame shift in HBB mRNA; however, detection of the mutant transcript and/or protein has been controversial. In genotypically identical relatives, a milder phenotype can sometimes be observed, while others have severe anemia. It has been suggested that these phenotypic differences may be mediated by variations in destruction of mutant HBB transcripts by nonsense mediated decay .
Materials: We studied a Dominican man with severe microcytic hypochromic anemia without iron deficiency, suggestive of thalassemia, and his family. We performed a detailed erythrocyte evaluation, including morphologic and hemoglobinopathy evaluations, globin sequencing, hemoglobin (Hb) stability evaluation, and band-3 testing for red blood cell (RBC) membrane abnormality, and analyzed DNA for mutations of genes encoding for RBC membrane proteins. We also performed in vitro expansion of erythroid progenitors, examined erythroid differentiation and proliferation, and searched for mutant HBB transcripts.
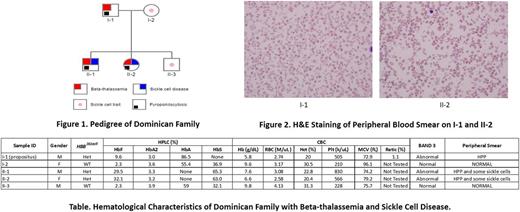
Results: The propositus was found to have a novel beta-thalassemia mutation, HBB282delT encoding a Cys93fs (frame shift). He also had Heinz bodies and was positive for an unstable Hb (by isopropanol test), but had no electrophoretically detectable mutant Hb, and had absolute reticulocytopenia (Table). Peripheral blood had a typical hereditary pyropoikilocytosis (HPP) morphology (Figure 1), confirmed by band-3 testing. Family studies (Table) showed that the situation was more complex. His asymptomatic wife and one son had sickle cell trait (Table), and two other children inherited both the HBB282delT mutation and a Hb S mutation and were positive for band-3 deficiency testing. Their RBCs had typical HPP morphology, along with sickle cells (Figure 2), and had high HbF levels (Table). Reticulocytes were not available for detection of mutant transcripts by RNA analysis; we therefore performed in vitro expansion of erythroid progenitors in a 3-week liquid erythroid culture model (Gaikwad, et al, Exp Hemat, 2007). We detected mutant HBB282delTtranscripts leading to a frameshift in exon 2, codon 93, where 9 new codons downstream from the mutation were encoded. The mutant transcript was present in equal amounts with the HBB transcript from the wild allele. Analysis of erythroid proliferation and differentiation revealed a marked decrease in proliferation (as determined by number of glycophorin-positive cells) and decreased differentiation (as evaluated by temporal changes of glycophorin and transferrin receptor positivity and intensity) in the propositus and two children with HBB 282delT. Hemoglobinization of their maturing erythroid progenitors was not discernible in 14-day cultures, in contrast to his wife, the asymptomatic son and the controls who had readily discernible hemoglobinized (red) progenitors. By day 21 of culture, he and the 2 affected children had only faintly hemoglobinized cells that appeared pink rather than red, and the erythroid progenitors of the propositus had minimal differentiation. None of the affected individuals had a SPTA1 exon 2 mutation, the most common missense mutations leading to the HPP phenotype.
Discussion: We describe a novel HBB mutation associated with severe anemia, reticulocytopenia, and impaired erythropoiesis with delayed erythroid maturation and impaired hemoglobinization. The presented data suggest that the mutant protein is unstable and presumably toxic to erythroid progenitors. The mutant protein is not identifiable by routine hemoglobin studies and its presence will be evaluated by studies of precipitated hemoglobin (Heinz bodies and hemoglobin precipitates after isopropanol exposure). Effects of the mutant protein on maturation and differentiation can be assessed by RNAseq of erythroid progenitors compared to normal controls of the same stage of erythroid differentiation. The molecular basis of HPP is being evaluated by DNA sequencing of targeted genes encoding RBC membrane proteins and comparing their potential functional relevance to the HPP phenotype.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal